Our work in exploratory and phase I, II & III pharmaceutical trials focuses on the automation and quantification of manual scoring and measurement systems currently used in musculoskeletal clinical studies to reduce timescales and cohort sizes by up to half.
CROs and pharmaceutical companies use Imorphics machine learning algorithms to automatically identify bony and soft tissue anatomy in 3D from MR or CT images, and are able to generate measurements that are highly reproducible compared to the semi-quantitative image reading that relies on human interpretation. The improved sensitivity of these measures can halve the numbers of patients needed to show a clinical effect.
In contrast to categorical scoring systems, continuous quantitative measures of distance, area or volume allow for the use of more powerful statistical methods. Automation means that analysis can be scaled up to large data sets of images with a rapid turnaround time, enabling data-based critical decision points in both retrospective and prospective clinical trials.
In addition to our work on automated scoring methods, we have produced completely novel 3D imaging biomarkers that have demonstrated superior responsiveness to the standard measures. These biomarkers are based on two important principles of our machine learning approach: a complete numerical description of anatomical shape; and the production of thousands of true corresponding landmark points on each of these shapes. This enables the completely objective comparison of 3D anatomy between time points or patients, uncovering exciting and important new insights into disease progression.
Want to know more? Contact us now to talk about how we can help.
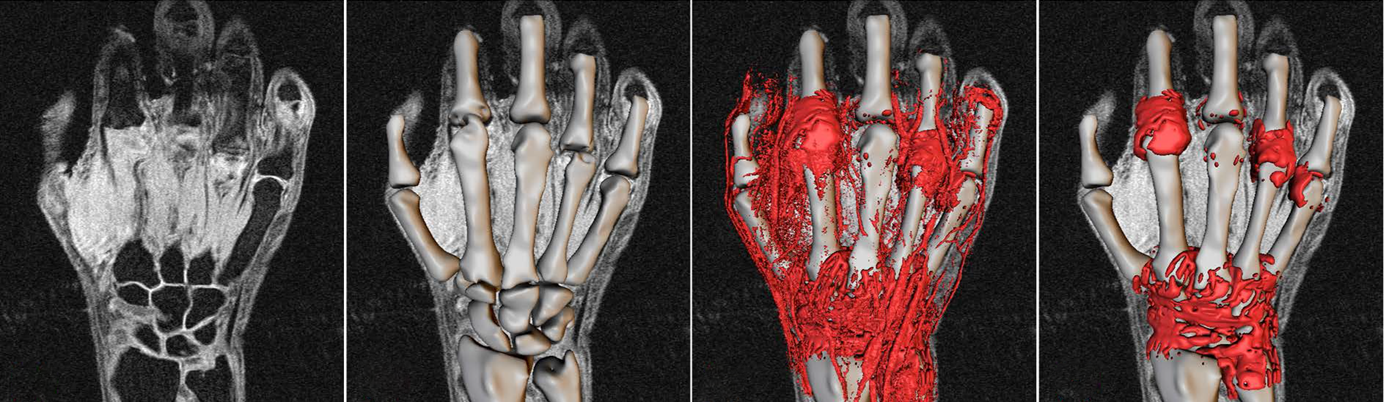
Rheumatoid Arthritis
Our technology provides users with a validated method of quantitative MRI measures based on the OMERACT RAMRIS scoring system.
The measurement of structural changes, as opposed to clinical changes, in subjects with rheumatoid arthritis (RA) is still largely based on x-ray imaging. However, x-rays are increasingly irrelevant in diagnosis and disease tracking because significant joint space narrowing and erosions are often not seen in patients who are undergoing early intervention treatment with an effective disease modifying antirheumatic drug (DMARD). In addition, it is unethical to conduct clinical trials without the standard of care (usually methotrexate) being used as an active comparator in order to prevent irreversible joint destruction – resulting in increased difficultly in powering a study that can demonstrate therapeutic effect.
To address this problem, we have produced a fully automated, quantitative version of the OMERACT RAMRIS semi-quantitative MRI scoring method. The resulting “RAMRIQ” system provides volumetric measures of synovitis, erosions and bone oedema in addition to joint space width. The system has shown good correlation with the RAMRIS scoring but with drastically improved sensitivity, reproducibility and speed. In addition, the continuous measures provided by RAMRIQ allows for more powerful statistical comparisons of subjects or time-points than the categorical output of RAMRIS. We have now extended the system to the measurement of tenosynovitis and are working to remove the need for Gd contrast agent.
Talk to us about how we can improve the speed and accuracy of your RA study.
Osteoarthritis
Our work in osteoarthritis has focused on the careful measurement of bone, articular cartilage and menisci.
The problem in clinical trials for a disease modifying osteoarthritis drug (DMOAD) is measuring very small changes in a disease that progress very slowly. Unless a measurement is both sensitive and highly reproducible, a very large patient cohort must be followed for several years. Due to the limitations of x-ray imaging in DMOAD trials, MRI has been identified as the most appropriate imaging modality to assess joint status in these studies because it can detect structural pathology in the bone, cartilage and soft tissues in addition to being used to image inflammation.
Imorphics statistical modelling of shape and appearance from MR images enables the precise measurement of established tissue change. In addition to precise regional cartilage analysis, we have shown that the bone itself is a highly responsive tissue that is often ignored in clinical trials. We have pioneered the use of 3D bone shape and area as sensitive and repeatable biomarkers of OA and have also produced methods of automated meniscal measurement, comparison of bone marrow lesion location and quantification of synovial inflammation at the knee.
Download the whitepaper or get in touch to find out how to apply Imorphics technology to your OA study.
